In a significant development for mental health treatment, the U.S. Food and Drug Administration (FDA) has expanded the approval of NeuroStar Transcranial Magnetic Stimulation (TMS) therapy to include patients aged 15 and up for the treatment of major depressive disorder (MDD). This groundbreaking decision marks a crucial step forward in addressing the growing concern of adolescent depression while continuing to provide effective treatment options for adults.
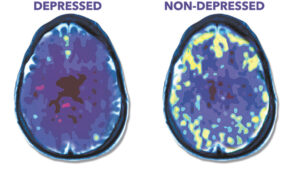
NeuroStar TMS, developed by Neuronetics, Inc., is a non-invasive treatment that uses magnetic fields to stimulate specific areas of the brain associated with mood regulation. The therapy has been FDA-approved for adult depression since 2008, but this recent expansion to include younger patients opens up new possibilities for treating depression in adolescents who may not have responded well to traditional treatments.
The decision to extend the approval to patients as young as 15 comes after rigorous clinical trials demonstrated the safety and efficacy of NeuroStar TMS in adolescent populations. These studies showed promising results, with many young patients experiencing significant improvement in their depressive symptoms and overall quality of life.
Depression among teenagers has been on the rise in recent years, with the COVID-19 pandemic exacerbating the issue. According to the National Institute of Mental Health, an estimated 3.8 million adolescents aged 12-17 in the United States had at least one major depressive episode in 2021. The approval of NeuroStar TMS for this age group provides a much-needed alternative for those who have not found relief through traditional treatments such as psychotherapy and medication.
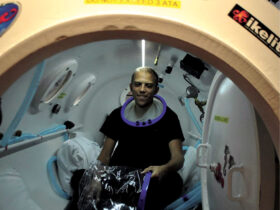
One of the key advantages of NeuroStar TMS is its non-invasive nature and relatively mild side effect profile compared to some pharmaceutical interventions. The treatment involves the patient sitting comfortably in a chair while a magnetic coil is placed against their head. The coil delivers magnetic pulses to stimulate nerve cells in the dorsolateral prefrontal cortex, an area of the brain involved in mood regulation. Each session typically lasts about 20-30 minutes, and a full course of treatment usually consists of 5 sessions per week for 4-6 weeks.
Parents and healthcare providers alike have expressed enthusiasm about this new option for adolescent depression treatment. Dr. Sarah Thompson, a child and adolescent psychiatrist, commented, “Having NeuroStar TMS available for our younger patients is a game-changer. It offers hope to those who haven’t responded well to other treatments and can be a crucial tool in preventing the long-term impacts of untreated depression in adolescents.”
The expansion of NeuroStar TMS to younger age groups also highlights the importance of early intervention in mental health treatment. Addressing depression in adolescence can potentially prevent more severe mental health issues in adulthood and improve overall life outcomes. By offering an effective treatment option earlier in life, healthcare providers can help set young patients on a path toward better mental health and well-being.
However, it’s important to note that while NeuroStar TMS has shown promising results, it is not a one-size-fits-all solution. The treatment is typically recommended for patients who have not achieved satisfactory improvement from antidepressant medications. As with any medical treatment, the decision to use NeuroStar TMS should be made in consultation with a qualified healthcare provider, taking into account the individual patient’s medical history, symptoms, and overall treatment plan.
The approval of NeuroStar TMS for ages 15 and up also underscores the evolving landscape of mental health treatment. As our understanding of the brain and its functions continues to grow, new technologies and approaches are being developed to address mental health disorders more effectively. This progress offers hope to millions of people struggling with depression and other mental health conditions.
As the medical community continues to embrace innovative treatments like NeuroStar TMS, it’s likely that we’ll see further advancements in the field of mental health care. The expansion of this treatment to younger age groups is just one step in the ongoing effort to improve mental health outcomes for people of all ages.
In conclusion, the FDA’s approval of NeuroStar TMS for patients aged 15 and up represents a significant milestone in the treatment of depression. By offering a non-invasive, effective option for both adolescents and adults, this therapy has the potential to transform lives and contribute to better mental health outcomes for millions of individuals struggling with depression. As research continues and technology advances, we can look forward to even more innovative and effective treatments in the future, bringing hope to those affected by mental health disorders.
Empowering Your Journey to Lasting Recovery
(239) 946-4131
www.zionhealing.com/fortmyers
Schedule a free consultation
9405 Cypress Lake Dr, Suite #2
Fort Myers, FL 39919