 According to the Asthma and Allergy Foundation of America, 26 million people have asthma, and allergic asthma is the most common type affecting 60% of sufferers. Allergic asthma has the same symptoms as asthma but is the result of what the body is having an allergic reaction to, which causes bronchial inflammation. Allergies can trigger the body’s immune response through skin absorption and contact, inhalation, ingestion, infusions or injections.
According to the Asthma and Allergy Foundation of America, 26 million people have asthma, and allergic asthma is the most common type affecting 60% of sufferers. Allergic asthma has the same symptoms as asthma but is the result of what the body is having an allergic reaction to, which causes bronchial inflammation. Allergies can trigger the body’s immune response through skin absorption and contact, inhalation, ingestion, infusions or injections.
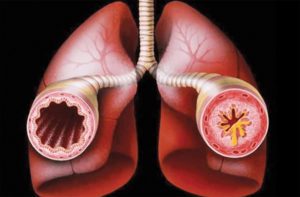
When a substance enters the body like dust mites (a common allergen), the antibodies, which are proteins bind to the allergen to defend itself. This causes an immunoglobin E (IgE) release. The E stands for Eosinophils. If too much IgE is in the body, it will produce allergic reactions like hives, itching eyes, nasal congestion, vomiting, a swollen mouth or anaphylaxis to name a few. It can also trigger asthma, which is a narrowing of the bronchial system and inflammation of the lungs, which makes it difficult to breathe. Too many eosinophils will trigger a white blood cell response and cause asthmatic wheezing, shortness of breath, coughing and in severe cases, the inability to breathe.
To control allergic asthma, the doctor must determine precisely what the allergens are, and treat those, along with managing the patient’s asthma. A blood test can be given to determine how many eosinophils are in the blood and their trigger. Once this is established, to control allergic asthma, certain biologics are preferred by pulmonologists like XOLAIR, FASENRA, and NUCALA.
XOLAIR
XOLAIR is the only FDA-approved biologic drug to treat allergic asthma patients 6 years of age and older, and unlike other medications, XOLAIR is specifically designed to treat allergic asthma by working on the underlying cause of allergic asthma. It captures the IgE in the body that may cause symptoms that can lead to an attack. XOLAIR is not inhaled, not a steroid, and not something you take daily. It has over 15 years of real-world experience helping patients with allergic asthma. XOLAIR has been prescribed for over 250,000 patients with allergic asthma. XOLAIR is a prescription medicine used to treat appropriate allergi asthma patients, and It is administered by injection, given under the skin every 2 or 4 weeks by a nurse or doctor who is prepared to manage anaphylaxis, which can be life-threatening. 1
FASENRA
FASENRA helps prevent severe asthma attacks. It has been clinically proven to reduce the occurrence of severe asthma attacks by up to 51%. In most patients it improves lung function, making it easier to breathe, and it reduces oral steroid use in people taking them every day by 75%. FASENRA is an add-on maintenance treatment for patients aged 12 and older with severe eosinophilic asthma. It is not an inhaler or a steroid; however, it is injected given by a healthcare provider every 8 weeks after the first 2 months.2
NUCALA
NUCALA is an add-on, prescription maintenance treatment for patients 12 and older with severe eosinophilic asthma. NUCALA is not used to treat sudden breathing problems. It is designed to reduce the number of eosinophils in the blood, and helps patients gain asthma control, as well as reduces the use of oral steroids like prednisone. NUCALA works over time, so patients may not feel immediate improvement due to its cumulative effect. It’s important to receive your injection of NUCALA once every four weeks as prescribed, even if you’re feeling better.3
If you or someone you know suffers from allergic asthma, please seek medical treatment as it can save lives.
To schedule an appointment with a pulmonary specialist, please call Pulmonary, Critical Care and Sleep Medicine Specialists of SWFL today at (239) 985-1925 or ask your primary care physician refer you to their office.
Pulmonary, Critical Care and Sleep
Medicine Specialists of SWFL
7335 Gladiolus Drive
Fort Myers, FL 33908
(239) 985-1925
www.breatheeasyswfl.com