By Dr. Alexandra Konowal –
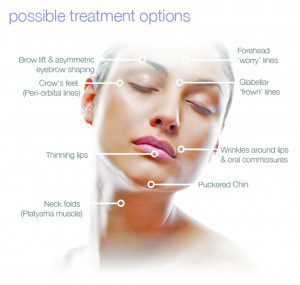 BOTOX® Cosmetic is the first and only FDA-approved treatment indicated for the temporary improvement in the appearance of moderate to severe frown lines in adult patients aged 65 years or less. BOTOX® Cosmetic is a purified protein produced by the Clostridium botulimum bacterium. It works by temporarily reducing the contractions of the muscles that cause persistent frown lines that have developed on the forehead, between the brows, as well as the crowsfeet from squinting over the years. It has been used to treat conditions caused by overactive muscles in over 1 million patients worldwide for more than 11 years.
BOTOX® Cosmetic is the first and only FDA-approved treatment indicated for the temporary improvement in the appearance of moderate to severe frown lines in adult patients aged 65 years or less. BOTOX® Cosmetic is a purified protein produced by the Clostridium botulimum bacterium. It works by temporarily reducing the contractions of the muscles that cause persistent frown lines that have developed on the forehead, between the brows, as well as the crowsfeet from squinting over the years. It has been used to treat conditions caused by overactive muscles in over 1 million patients worldwide for more than 11 years.
BOTOX® is indicated for the treatment of cervical dystonia in patients with neck pain, blepharospasm in people 12 years of age and above, as well as migraine headaches. It has also been found to aid patients with hyperhidrosis, or excessive sweating of the underarms, hands and feet.
No anesthesia is required for BOTOX® treatments, although some patients choose to have the desired area numbed with a cold pack or anesthetic cream. The entire procedure takes approximately 10 minutes, with the number of injections depending on the amount and severity of the wrinkles or frown lines. Patients can resume normal activities afterwards, since there is no recovery time needed. An improvement in the frown lines and wrinkles is noted within days and can last up to four months. Results may vary and touch-ups may be necessary for deeper furrows. If you do not continue the treatments, the frown lines will gradually return to their appearance before treatment.
BOTOX® Cosmetic should bot be used in the presence of infection at the proposed site of injection, in individuals with known hypersensitivity to any ingredient in the formulation and in patients with neurological disorders, such as ALS, myasthenia gravis or Lambert-Eaton syndrome. Patients who are pregnant or breastfeeding should not undergo treatment with BOTOX® Cosmetic. The most common side effects include redness and bruising at the site of injection, headache, temporary eyelid droop, flu syndrome and nausea.
BOTOX® Cosmetic has been used to treat a million people in the United States since it was approved by the FDA in 2002. In clinical trials, nearly 90% of men and women surveyed rated the improvement in the appearance of frown lines between their brows as moderate to better one month after treatment.
Juvederm® is an FDA-approved cosmetic dermal filler made from hyaluronic acid. Hyaluronic acid is a naturally occurring substance in your skin that helps to hydrate and add volume. Hyaluronic acid in your skin decreases with age and over time, and this loss of support and volume leads to the formation of wrinkles and folds – especially in your lower face. Juvederm® corrects these processes by returning hyaluronic acid in your skin.
Juvederm® can be used to “fill in” wrinkles, furrows and depressions around the nose and mouth, including the nasolabial folds, marionette lines, and smile lines. Juvederm® temporarily adds volume underneath your skin to restore a smoother appearance to your face. It can also be used to plump the lips and decrease those smokers lines that you can get even without smoking. It can reduce eye bags, and fill the cheeks, making you look younger and refreshed instantly.
RADIESSE® dermal filler is an injectable filler for facial folds and wrinkles. It contains microspheres of Calcium Hydroxyapatite, a biocompatible, biodegradable material. It is similar in composition to the mineral portion of teeth and bone and has a history of safe use in dental and orthopedic applications.
RADIESSE® is used to fill moderate to severe facial wrinkles and folds, such as nasolabial folds, the creases that extend from the corner of your nose to the corner of your mouth. Once injected, RADIESSE® dermal filler immediately adds fullness to your face giving you a visible result at the first treatment session. RADIESSE® filler contains microspheres made of natural material called Calcium Hydroxyapatite in a water based gel carrier. Studies have shown that six months after treatment with RADIESSE® dermal filler, there is an increase in collagen production to the areas of injection.