 Being diagnosed with any disease can be scary, even devastating. For those who have been diagnosed with Parkinson’ Disease (PD), it can be especially difficult to come to
Being diagnosed with any disease can be scary, even devastating. For those who have been diagnosed with Parkinson’ Disease (PD), it can be especially difficult to come to
terms with because the diagnosis is usually clinical. Until the last decade, the only diagnosis came from careful observation by a neurologist. In fact, for many that is still the only diagnosis given and many are left questioning if they really have the disease or if it could be something different.
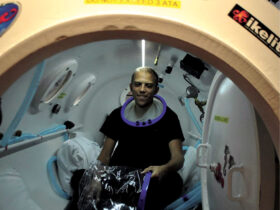
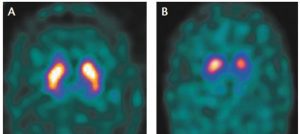
In 2011, the Food and Drug Administration (FDA) approved an imaging test to help diagnose PD. In this test, a radioactive tracer, loflupane 123 I, also known as DaTscan, is injected into the blood, where it circulates around the body and makes its way into the brain. It attaches itself to the dopamine transporter, a molecule found on dopamine neurons. Several hours after the tracer has been injected, special imaging equipment scans the head to detect the presence of DaTscan.1
According to a press release made on November 3, 2022, by GE Healthcare, the DaTscan’s manufacturer, it has also been approved by the FDA for use in diagnosing Dementia with Lewey Bodies. The press release states:
“GE Healthcare’s DaTscan has been approved by the U.S. Food and Drug Administration (FDA) for use in patients with suspected Dementia with Lewy Bodies (DLB). This new indication is in addition to its use with single photon emission computed tomography (SPECT) imaging to visualize dopamine transporters (DaTI in the brains of adult patients with suspected Parkinsonian syndromes. With the expanded indication, DaTscan is now available to more patients, including those with suspected DLB, in the United States.
The clinical signs and symptoms of DLB can be atypical and overlap with other forms of dementia, leading to up to 70% of patients with DLB being misdiagnosed, often as having Alzheimer’s Disease. This new indication enables clinicians to use DaTscan to help differentiate DLB from other forms of dementia. Early and accurate diagnosis of DLB can help ensure specific appropriate treatment and specialized care for patients, while enabling them and their caregivers to more effectively manage the
disease and plan for the future. Approximately one in five patients with dementia suffers from DLB, making it the second most common form of degenerative dementia after Alzheimer’s Disease.
Professor James E. Galvin, MD, MPH, Consultant, University of Miami Miller School of Medicine, U.S., said: “Misdiagnosis is a significant issue for those patients with suspected Dementia with Lewy Bodies, causing untold anxiety for the patient and family as well as potentially placing the patient at higher risk of adverse events due to delayed diagnosis. The label expansion for DaTscan moves patients a step closer to an earlier, more accurate, diagnosis which is beneficial for them and their families, setting them on the right treatment path sooner and helping to avoid medications and treatments that could be potentially harmful.”
Mark Hibberd, Chief Medical Officer for GE Healthcare Pharmaceutical Diagnostics, said: “More than one million doses of DaTscan have already been used around the world in the clinical evaluation of Parkinsonian syndromes. We have built on our scientific and medical leadership with DaTscan to pave the path for this new indication, that supports our customers and their patients with more accurate diagnoses of DLB.”
The approval of the use of DaTscan in DLB is the culmination of significant work, including clinical trials, compilation and analysis of data and collating evidence for submission to the U.S. FDA, all demonstrating GE Healthcare’s continued commitment and investment in this space. Earlier this year, GE Healthcare announced plans to bolster its Molecular Imaging neurology portfolio by complementing DaTscan with two pipeline radiopharmaceuticals, one for Positron Emission Tomography (PET) and another for SPECT, aiming to offer customers, in both clinical and research settings, a wider choice of diagnostic tracers to help evaluate adult patients with suspected Parkinson’s syndrome’s.”
Radiology Associates of Venice, Englewood, and Sarasota strive to combine years of physician education and experience with cutting edge technology to provide excellent radiologic diagnosis and intervention.
RAVE is a radiology practice which has been active for over fifty years. We currently consist of 10 board certified radiologists, many of which have postgraduate fellowships with subspecialty training. All of whom have years of full-time experience.
There are over 120 health care professionals working with us to provide the best possible radiologic services in Sarasota County. In addition to our personnel, we have some of the best radiologic imaging devices available. Superior visualization helps us make your diagnoses accurate and timely, preventing potentially dangerous delays in initiation of your therapy. At RAVE, you can have confidence you’re getting top notch professional assistance in your diagnosis and care.
Have your physician send you to our office in Venice for DaTscan testing if it’s suspected that you or your loved one might be experiencing dementia or Parkinson’ symptoms.
RAVE IMAGING
www.raverad.com
VENICE
512-516 S. Nokomis Ave
Venice, FL 34285
941-488-7781
Hours: 8:00am-10:00pm
ENGLEWOOD
900 Pine Street
Englewood, FL 34223
941-475-5471
Hours: 8:00am-10:00pm
SARASOTA
3501 Cattlemen Road
Sarasota, FL 34223
941-342-RAVE (7283)
Hours: 8:00am-10:00pm
1. Gilbert, D.R. (2022) What is a DaTscan and should I get one?,
American Parkinson Disease Association. Available at:
https://www.apdaparkinson.org/article/what-is-a-datscan-and-
should-i-get-one/.